Background
Janus kinase inhibitors are actively investigated for treating various hematological, rheumatological, gastrointestinal and dermatological indications. Thus, we have initiated a living systematic review to define the toxicity profile of JAKi agents. Here, we report an up-to-date assessment of toxicity profile of JAKi in patients with myelofibrosis.
Methods
MEDLINE, EMBASE and CENTRAL were systematically searched from each database's inception through June 13, 2023, to identify phase I/II/III clinical trials assessing JAKi in myelofibrosis reporting adverse events. The main outcomes of interest included incidence rates of all-cause and treatment-related grade ≥ 3, grade 5 and all-grade AEs of interest (infections, hematological, gastrointestinal, and cardiovascular adverse events). Incidence rates (incidence per 100-person-years) for AEs of interest were calculated at the level of each trial. A random-effects meta-analysis was conducted to pool the incidence rates of AEs using an inverse-variance approach.
Results
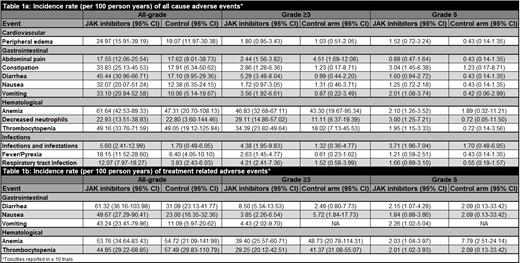
The literature search identified 2044 studies from which 28 clinical trials (33 references) met the inclusion criteria - 4 (14%) trials were phase I, 4 (14%) trials were phase I/II, 13 (46%) trials were phase II, and 7 (25%) trials were phase III. A total of 4505 participants received JAKi and were eligible for analysis. All-cause and treatment-related hematological toxicities were reported in 28 (100%) and 18 (64%) trials, all-cause and treatment-related gastrointestinal toxicities were reported in 28 (100%) and 18 (64%) trials, all-cause and treatment-related cardiovascular toxicities were reported in 20 (71%) and 12 (43%) trials whereas all-cause and treatment-related infections were reported in 26 (93%) and 14 (50%) trials, respectively. The incidence rate per 100-person years for all-cause and treatment related adverse events of interest are summarized in Table 1a and 1b respectively. For treatment-related AEs, the incidence of grade ≥ 3 diarrhea with JAKi is 8.50 (95% CI: 5.34-13.53) events per 100-person years. The incidence of treatment-related grade ≥ 3 anemia and thrombocytopenia is 39.40 (95% CI: 25.57-60.71) and 29.25 (95% CI: 20.12-42.51) events per 100-person years respectively. The incidence of treatment-related grade 5 diarrhea with JAKi is 2.15 (95% CI: 1.07-4.29) events per 100-person years whereas the incidence of treatment-related grade 5 anemia and thrombocytopenia is 2.03 (95% CI: 1.04-3.97) and 2.01 (95% CI: 1.02-3.93) events per 100-person years respectively. For all-cause AEs, the incidence of grade ≥ 3 nausea, vomiting and diarrhea with JAKi is 1.72 (95% CI: 0.97-3.05), 3.56 (95% CI: 1.92-6.61) and 5.29 (95% CI: 3.48-8.04) events per 100-person years respectively. The incidence of all-cause grade ≥ 3 infections with JAKi is 4.38 (95% CI: 1.95-9.83) events per 100-person years whereas the incidence of all-cause grade ≥ 3 respiratory tract infections with JAKi is 4.21 (95% CI: 2.41-7.36) events per 100-person years. The incidence of all-cause grade ≥ 3 anemia, neutrophil decrease and thrombocytopenia is 46.83 (95% CI: 32.68-67.11), 29.11 (95% CI: 14.86-57.02) and 34.39 (95% CI: 23.82-49.64) events per 100-person years respectively. Moreover, the incidence of all-cause grade 5 nausea, vomiting and diarrhea with JAKi is 1.25 (95% CI: 0.72-2.18), 2.01 (95% CI: 1.08-3.74) and 1.60 (95% CI: 0.94-2.72) events per 100-person years respectively. In addition, the incidence of all-cause grade 5 infections and infestations is 3.71 (95% CI: 1.96-7.04) events per 100-person years with the incidence of grade 5 respiratory tract infections being 1.66 (95%CI: 0.89-3.10) events per 100-person years. Furthermore, the incidence of all-cause grade 5 anemia, neutrophil decrease and thrombocytopenia is 2.10 (95% CI: 1.26-3.52), 3.00 (95% CI: 1.25-7.21) and 1.95 (95% CI: 1.15-3.33) events per 100-person years respectively.
Conclusions
This report of living systematic review provides the best current evidence on toxicity profile of JAKi in patients with myelofibrosis. Current evidence suggests an overall higher incidence of all grade and grade ≥ 3 treatment related gastrointestinal adverse events in patients receiving JAKi. This living review will be updated as soon as new data becomes available.
Disclosures
Palmer:morphosys: Consultancy, Other: Money went to institution; Jubliant: Consultancy; Incyte: Consultancy, Other: Money went to the institution; Sierra Oncology: Consultancy, Other: Money went to Institution; CTI BioPharma Corp.: Consultancy, Honoraria, Other: Money went to institution.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal